Current commercially available lithium-ion batteries have a relatively high capacity. But they have a limitation: their charging-discharging cycle is slow. In addition, they contain organic electrolytes and other materials that can be hazardous and flammable, meaning they require careful handling and disposal.
Now, scientists at the departments of Physics and Chemistry at Imperial College London have designed a new battery prototype using salt water and materials that are non-toxic. Most fascinating is this prototype charge quickly, paving the way for new types of battery.
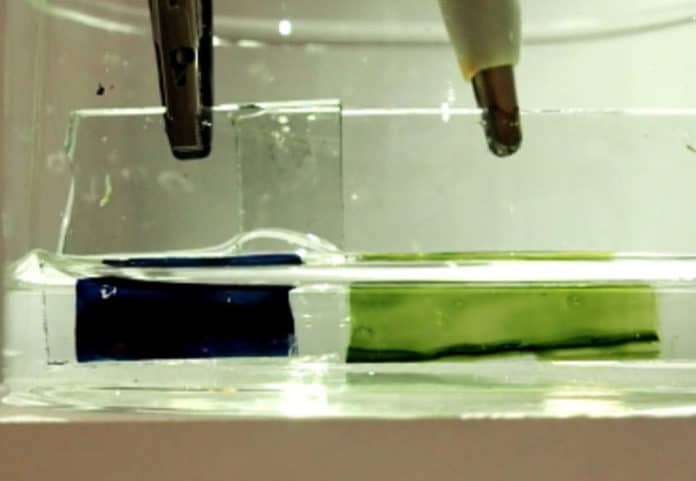
The prototype changes its color while it charges and could potentially be applied to existing battery technologies to create new devices for energy storage, biological sensing, and smart color-changing materials.
The prototype uses thin films of specially designed plastics and simple salt water instead. It also can hold less charge than conventional lithium-ion batteries. Due to its changing color during charging, the user can read easily about its charge state.
Co-lead author Dr. Alexander Giovannitti, who worked on the project while at the Departments of Physics and Chemistry at Imperial, said: “The materials we used to create the battery prototype could potentially be made at low cost and combined with the use of non-toxic and non-flammable water-based electrolytes. This approach could be a viable route to develop recyclable batteries.”
On a larger scale, when renewable technologies like solar or wind are used as part of a national or local grid, they can only provide energy intermittently. A battery system that could store this energy quickly, but also give it back to the grid when needed, would be valuable in keeping supply steady.
The team says their prototype would need more work to be suited to these areas, but that the principles behind its design could be applicable to a wide range of energy storage devices in development.
The breakthrough comes from the design of polymer materials that can take up and release positive or negative ions from salt water, quickly and reversibly without degrading. These ions are attracted to electrodes of opposite charge when the device is charging.
Water-based batteries are beneficial and non-toxic, but it has been difficult to get the ions in water to be reversibly exchanged with the electrodes.
Co-lead author Dr Davide Moia, who completed the work while at the Department of Physics at Imperial, said: “Using salt water gets rid of toxicity and flammability concerns, but it has not been easy to use as it can limit the amount of energy you can get in and out of a device compared to other organic electrolytes.
“We now want to test how far this limit can be pushed. We have compensated for lower performance with a safer combination of materials but improving the performance could open up the path to whole new types of viable energy storage devices that are also safe and sustainable.”
The team got around this by designing side-chains to attach to the conducting polymer ‘backbones’. By using polar materials for the side chains, they could create electrodes with high affinity to water.
With this principle, they were able to create positive and negative electrodes that can host their opposite ions from the water – and they had the ingredients for a battery. As the polymer backbones were already flexible – expanding and contracting while the battery charged and discharged – then no additives were needed.
Co-lead author Dr Davide Moia, who completed the work while at the Department of Physics at Imperial, said: “Using salt water gets rid of toxicity and flammability concerns, but it has not been easy to use as it can limit the amount of energy you can get in and out of a device compared to other organic electrolytes.
“We now want to test how far this limit can be pushed. We have compensated for lower performance with a safer combination of materials, but improving the performance could open up the path to whole new types of viable energy storage devices that are also safe and sustainable.”
The study is published in the journal Energy & Environmental Science.